SKEDSOFT
SPONTANEOUS EMISSION: It is well known that there are various energy levels in an atom. Ground state of the atom is the minimum energy state and it is the most stable state. When the atom gets suitable thermal energy, its valence electron (say of energy E1) jumps to higher energy level (say to energy E2) called excited level. Electrons in this level are also called atoms in its excited state. Life time of electron in the excited state is very small, of the order of 10–8 sec, hence the electron within this time falls back to lower energy level E1 by emitting a radiation. This process is called spontaneous emission. The frequency, ν of the emitted radiation is given by
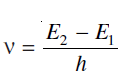
where h is the Planck constant. We also say that in this transition a photon of energy hν is emitted. If there are large number of atoms in the upper energy level then the emitted radiations will have randomly different initial phases and directions and the emitted radiations will be incoherent.
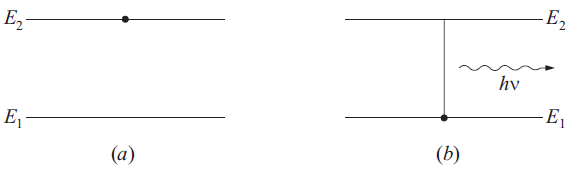
fig..(1)(a) Initial state, (b) Final state
STIMULATED EMISSION: If an atom is in an excited state of energy E2 and an incident photon of suitable energy (hν = E2 – E1) may cause the atom (electron) to jump to lower energy state (≈ E1) emitting an additional photon of same frequency, same phase and in the same direction. Thus now two photons each of energy equal to E2 – E1 are present. This kind of transition is called stimulated emission of radiation. This is shown in Fig. 2.
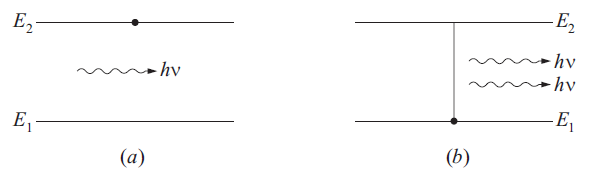
fig..(2)(a) Initial state (b) Final state
ABSORPTION OF RADIATION: If an atom in its ground state of energy E1 and radiation of suitable energy (hν = E2 – E1) is given such that the atom goes to excited state E2 i.e., its electron jumps from E1 level to higher energy state E2 by absorbing a quantum of radiation or photon. This kind of transition is called the absorption of radiation. This is shown in Fig. 3.
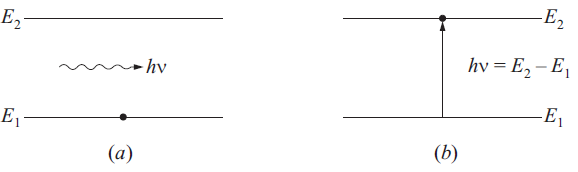
fig..(3)(a) Initial state (b) Final state