SKEDSOFT
Ferroelectricity: Ferroelectric crystal exhibits an electric dipole moment even in the absence of an external electric field. In the ferroelectric state the center of positive charge of the crystal does not coincide with the center of negative charge. The plot of polarizations versus electric field for the ferroelectric state shows a hysteresis loop. A crystal in a normal dielectric state usually does not show significant hysteresis when the electric field is increased and then reversed, both slowly. In ferroelectric materials the ionic susceptibility is sensitive to variations in temperature. In these substances, the static dielectric constant changes with temperature according to the relation
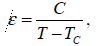
where C is a constant independent of temperature.
This behavior is valid only in the temperature range T > Tc. In the range T < Tc, the material becomes spontaneously polarized, i.e., an electric polarization develops in it without the help of an external field. (This phenomenon is analogous to the spontaneous magnetization which takes place in ferromagnetic materials.)
A phase transition occurs at the temperature Tc. Above the transition temperature, the substance is in the paraelectric phase, in which the elementary dipoles of the various unit cells in the crystal are oriented randomly. The dielectric constant is shown in Fig.1a. Below the transition temperature, the elementary dipoles interact with each other, and this gives rise to an internal field, which lines up the dipoles. The direction of this field and the associated polarization lie in a certain favorable orientation in the crystal. Figure 9b shows the variation of the spontaneous polarization P with temperature for T < Tc. This polarization increases gradually as the temperature is lowered.
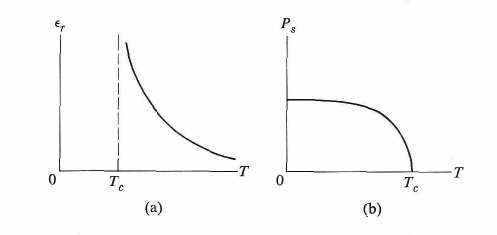
Fig. 1(a) Dielectric constant e versus T in a ferroelectric substance, (b) Spontaneous polarization PS versus T in a ferroelectric substance.
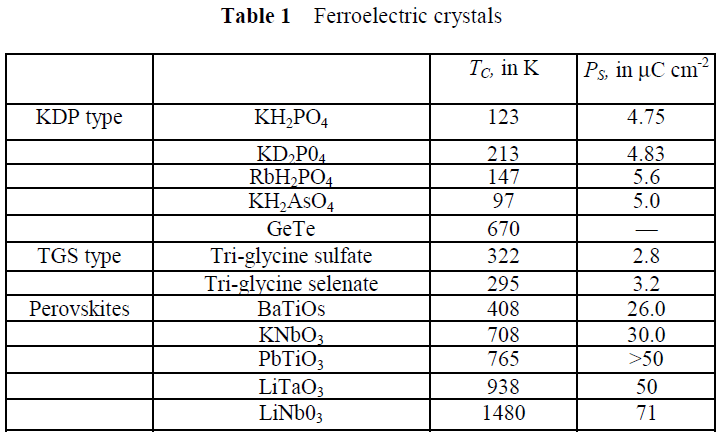
Table 1 lists some crystals commonly considered to be ferroelectric, along with the transition temperature or Curie point TC at which the crystal changes from the low temperature polarized state to the high temperature unpolarized state. Thermal motion tends to destroy the ferroelectric order. Some ferroelectric crystals have no Curie point because they melt before leaving the ferroelectric phase. The table also includes values of the spontaneous polarization PS.
Ferroelectric crystals may be classified into two main groups: order-disorder or displacive. In ferroelectric materials exhibiting an order-disorder transition the displacements of atoms are about some double-well or multi-well configuration of sites in the paraelectric phase. In the ferroelectric phase the displacements are about an ordered subset of these wells. The orderdisorder class of ferroelectrics includes crystals with hydrogen bonds in which the motion of the protons is related to the ferroelectric properties, as in potassium dihydrogen phosphate (KH2PO4) and isomorphous salts.
In ferroelectric materials exhibiting a displacive transition the atomic displacements represent oscillations about a nonpolar site in the paraelectric phase. After a displacive transition (ferroelectric phase) the oscillations are about a polar site. The displacive class of ferroelectrics includes ionic crystal structures closely related to the perovskite and ilmenite structures. The simplest ferroelectric crystal is GeTe with the sodium chloride structure.