SKEDSOFT
which is the sum of the electronic, ionic, and dipolar polarizabilities, respectively. The electronic contribution is present in any type of substance, but the presence of the other two terms depends on the material under consideration.
The relative magnitudes of the various contributions in are such that in nondipolar, ionic substances the electronic part is often of the same order as the ionic. In dipolar substances, however, the greatest contribution comes from the dipolar part. This is the case for water, as we shall see.
Another important distinction between the various polarizabilities emerges when one examines the behavior of the ac polarizability that is induced by an alternating field. Figure 4 shows a typical dependence of this polarizability on frequency over a wide range, extending from the static all the way up to the ultraviolet region. It can be seen that in the range from ω = 0 to ω = ωd, where ωd (d for dipolar) is some frequency usually in the microwave region, the polarizability is essentially constant. In the neighborhood ωd, however, the polarizability decreases by a substantial amount.
This amount corresponds precisely, in fact, to the dipolar contribution αd. The reason for the disappearance of αd in the frequency range ω > ωd is that the field now oscillates too rapidly for the dipole to follow, and so the dipoles remain essentially stationary. The polarizability remains similarly unchanged in the frequency range from wd to wi and then drops down at the higher frequency. The frequency wi lies in the infrared region, and corresponds to the frequency of the transverse optical phonon in the crystal. For the frequency range w > wi the ions with their heavy masses are no longer able to follow the very rapidly oscillating field, and consequently the ionic polarizibility αi, vanishes, as shown in Fig. 4.
Thus in the frequency range above the infrared, only the electronic polarizability remains effective, because the electrons, being very light, are still able to follow the field even at the high frequency. This range includes both the visible and ultraviolet regions. At still higher frequencies (above the
electronic frequency ωe, however, the electronic contribution vanishes because even the electrons are too heavy to follow the field with its very rapid oscillations.
The frequencies ωd and wi, characterizing the dipolar and ionic polarizabilities, respectively, depend on the substance considered, and vary from one substance to another. However, their orders of magnitude remain in the regions indicated above, i.e., in the microwave and infrared, respectively. The various polarizabilities may thus be determined by measuring the substance at various appropriate frequencies.
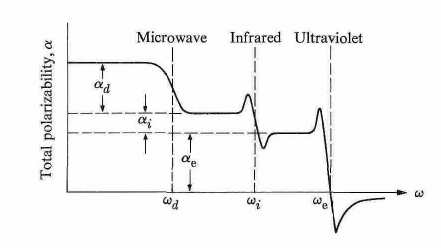
Fig.4 Frequency dependence of the several contributions to the polarizability.